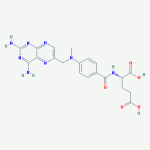
50-50, A Serious Film About a Young Man With a Rare Cancer
The movie, based in part on the true story of scriptwriter Will Reiser, surprised me by its candor. Actor Joseph Gordon-Levitt smoothly portrays Adam Lerner, who soon finds out he has cancer. The opening scene