Cervical Cancer Screening Update: on Pap Smears, Liquid-based Cytology and HPV
The latest issue of the Annals of Internal Medicine contains 2 noteworthy papers on cervical cancer screening. The first, a systematic review of studies commissioned by the USPSTF, looked at 3 methods for evaluating abnormalities in women over 30 years:
1. Conventional cytology (as in a Pap smear; the cervix is scraped and cells splayed onto a microscope slide for examination);
2. Liquid-based cytology (for LBC, the NHS explains: the sample is taken as for a Pap test, but the tip of the collection spatula is inserted into fluid rather than applied to slides. The fluid is sent to the path lab for analysis);
3. Testing for high-risk HPV (human papillomavirus). Currently 3 tests have been approved by the FDA in women with atypical cervical cells or for cervical cancer risk assessment in women over the age of 30: Digene Hybrid Capture 2 (manufactured by Quiagen), Cobas 4800 HPV (Roche) and Cervista HR HPV (Hologic); another Roche Diagnostics assay, Amplicor HPV, awaits approval.
These HPV assays use distinct methods to assess DNA of various HPV strains.
There’s a lot of jargon here, and I have to admit some of this was new to me despite my nearly-due diligence as a patient at the gynecologist’s office and my familiarity as an oncologist with the staging, clinical manifestations and treatment of cervical cancer. Who knew so many decisions were made during a routine pelvic exam about which manner of screening?
The main points I took away from this paper:
1. Liquid-based cytology is similar to conventional Pap smear cytology for detecting high-grade dysplasia (abnormal cells) and cervical cancer.
2. It seems that at some medical centers, and possibly overall, there’s a lower proportion of inadequate cell specimens when practitioners skip the slides and use the liquid method. This means that fewer women need be called back for another procedure.
3. Finding HPV sequences in the cervix yields many false positives, in terms of malignancy.
The researchers conclude that further studies are needed to sort out how HPV testing can improve or supplement cervical cancer screening. The main limitation is that many young women are infected with potentially cancer-causing strains of HPV, but most don’t get cervical cancer. When cervical cancer does develop that’s usually later on, a decade or longer after the relevant viral infection.
The second Annals article, a helpful narrative review, considers the practical implications of the above findings. The authors state that over 40 types of HPV can infect the cervix. They review that progression to cancer occurs along these 4 steps: HPV transmission, acute infection, persistent infection causing precancerous changes and eventually, in a subset of those infected, invasive cervical cancer.
Figure 1 is remarkably clear:
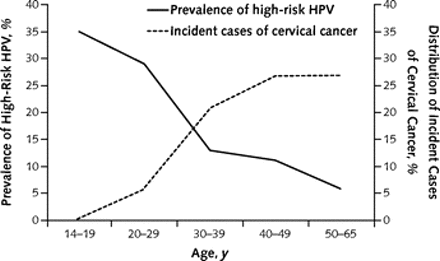
The graph shows that the prevalence of HPV infection is highest among teens and women in their early 20s, and decreases in older women. By contrast, the incidence of cervical cancer rises steadily in women over 30 years and remains elevated among women in their 40s. The authors show, separately, that the rate of cervical cancer in older women is low.
The central point is that high-risk HPV infection and associated inflammation of the cervix are common in young women, but cervical cancer is rare among those under 30 years. The investigators conclude that cervical cancer screening in women younger than 20 years may be harmful. They also state that evidence supports discontinuation of cervical cancer screening in most women who are over 65 years old.
—
Two asides on this otherwise non-bloggy topic –
It’s great that the Annals provides the full text of these papers open-access, free of charge to the public.
Amazing how well-accepted is the concept of some viruses causing cancer, today. This was a heretical idea 25 years ago in academic medicine; now it’s dogma.
—
Some additional points:
1. HPV testing should not be done routinely in women under 30. But for young women with ambiguous results on Pap, testing for “high risk” HPV can determine which women need further testing for precancerous lesions.
2. HPV testing should be done to detect “high risk” types of HPV which are known to cause cervical cancer. Though tests exist to detect “low risk” types of HPV (which cause genital warts but not cancer)there is no real indication to order these tests. http://journals.lww.com/greenjournal/Abstract/2011/07000/Low_Risk_Human_Papillomavirus_Testing_and_Other.2.aspx
3. Appropriate use of testing for “high risk” types of HPV can safely reduce the frequency of Pap/cervical cytology testing in women who test negative for HPV. Women who test positive for HPV are at increased risk for cervical cancer and need to be followed more closely.
Thanks, Jessica, for your input on this. Cervical cancer screening is becoming so complicated! Still, it’s a spectacular success in terms of reducing mortality from cervical cancer over the past 100 years.