FDA Approves New Assay for Her2 in Breast Cancer
This week the FDA approved a new assay for Her2 expression in breast cancer biopsies. The technology, Inform Dual ISH, is manufactured by Ventana Medical Systems, a Roche subsidiary.
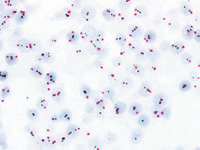
Inform Dual ISH works like this: technicians, typically working under the supervision of a pathologist, expose a tiny bit of a breast biopsy specimen, fixed on a microscope slide, to probes for Her2. This gene, normally found on human chromosome 17, is amplified in some breast cancer cells. The assay exploits an enzyme, linked to the genetic probe, which creates a color (in this case, red) upon exposure to a chemical. The system allows a pathologist, using a microscope, to “see” and measure the gene’s presence on chromosomes in cells of an ordinary biopsy sample.
What’s interesting about this in situ hybridization (ISH) kit is that it doesn’t require a fluorescent microscope for imaging. The Ventana probe generates a simpler, ordinary color signal that can be detected by a light microscope. Most commercial assays for Her2 use a method called immunohistochemistry (IHC); that technique relies on antibodies that bind Her2, a cell surface receptor that’s implicated in cancer cell signaling and growth. This and other ISH assays measure genes directly on the chromosomes; by contrast, IHC usually tests for protein.
Her2 is the molecular target for Herceptin, and there’s been considered discussion about how and where it might be accurately assessed. So the “readout” from this diagnostic test might inform a woman and her doctor in deciding whether or not she should receive treatment with Herceptin.
How pathologists evaluate breast biopsy specimens matters a lot, especially when you’re on the receiving end of a diagnosis and you’re choosing among treatments. In 2007, ASCO and the College of American Pathologists published guidelines on Her2 testing in the Journal of Clinical Oncology. These groups recently updated the recommendations. How this new assay will be received by these societies, I’m not sure.
A key question, in this author’s mind, is where the Her2 measurements take place, and whether women should rely on local labs’ assays – by whatever method – to determine the Her2-ness of their breast tumors.
—
Leave a Reply