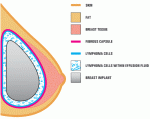
It’s a Pandora’s box, but one that needs be opened. The problem is that if we biopsy every abnormality – such as a minor thickening or fluid accumulation adjacent to a breast implant – we’ll hike up the costs and, more importantly, the complications associated: With every needle stick there’s a risk of infection, additional scar formation and more. On the other hand, you wouldn’t want to overlook a treatable, early-stage lymphoma. Women need to know of the risks of implants, which can only be determined if doctors thoroughly investigate these sorts of complications.
Posted in Breast Cancer, cancer treatment, Medical News, Oncology (cancer), Plastic and Reconstructive Surgery, Women's HealthTagged ALCL, anaplastic large cell lymphoma, anaplastic lymphoma, breast implants, FDA alert, informed consent, lymphoma, mastectomy, reconstructive surgery