FDA Approves Pertuzumab for Advanced, Her2+ Breast Cancer
We’re on a roll for new treatments of the Her2+ form of breast cancer. On Friday the FDA approved Pertuzumab, a monoclonal antibody, for advanced cases. As indicated, the drug would be given along with another monoclonal antibody, trastuzumab (Herceptin) and a chemotherapy, docetaxel (Taxotere) to patients with advanced breast tumors with high levels of Her2.
The new treatment’s brand name is Perjeta. Like Herceptin, this reagent works by attaching to the Her2 receptor on a cancer cell’s surface. But it differs by binding a distinct part of the molecule; its mechanism of action is said to complement that of Herceptin. You might recall that HER protein family members are complex signaling molecules that span cell membranes. Her1 is the Epidermal Growth Factor Receptor; it’s turned on when bound by its partner, or molecular ligand, Epidermal Growth Factor(EGF). The others are Her2, -3 and -4.
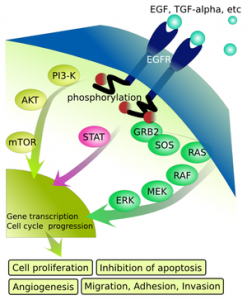
The science behind drugs that interfere with Her2 receptors and signaling is nicely summarized in a recent, open-access Nature Reviews Clinical Oncology article. Herceptin binds a particular segment of Her2 on the outside of the cell; this leads to failed signaling on the inside, including cell division signals, and causes cell death by several mechanisms. Pertuzumab binds a distinct segment of Her2 in such a way that it can’t form a complex with the related Her3 molecule; this interaction is needed for Her2 to stimulate cell growth.
The FDA’s approval rests largely on results of the CLEOPATRA (Clinical Evaluation of Pertuzumab and Trastuzumab) study, published earlier this year in the NEJM. In that Phase III study, just over 800 patients were randomly assigned to receive a standard regiment – Herceptin in combination with Taxotere plus a placebo infusion, or the Herceptin-Taxotere combination plus Pertuzumab.
The patients who received Pertuzumab did better in terms of Progression Free Survival (PFS, 18.5 months vs. 12.4 months; this difference holds strong statistical merit). There is a trend, also, in terms of Overall Survival: at a median follow-up point of 19.3 months, there were more deaths in the placebo group. But a statistically significant difference was not reached. Toxicity was reported as “generally similar” in the two groups, but there was more diarrhea, dry skin and rashes among those who got Pertuzumab (Table 3). Heart problems, a known toxicity of the Herceptin-Taxotere regimen, were slightly less common with Pertuzumab. Hair loss, presumably from the chemotherapy part of the regimen, was common in both groups.
One curious thing I noticed, in re-reading the January report, is that although the median age for both patient groups was 54 years, the control patients ranged from 27 – 89 in age; those who got Pertuzumab ranged from 22 – 82 years. Although the younger “shift” of Pertuzumab-receiving patients relative to the controls is unlikely to affect the PFS, it’s odd to include an 89 year old patient on an experimental protocol involving infusions of two monoclonal antibodies along with chemo.
This is a super-costly regimen. Like Herceptin, and like the experimental compound antibody, DM1, about which I wrote last week, Perjeta is manufactured by Genentech. As detailed by Andrew Pollack in the NY Times: the wholesale price for Perjeta will be $5,900 per month for a typical woman; Herceptin costs $4,500 per month. So we’re talking about a treatment in which the monoclonal antibodies alone cost over $10K per month. “A typical 18-month course of treatment would be more than $187,000,” he indicated. But if you add on the costs of the Taxotere, drugs like Benadryl and Decadron to minimize allergic reactions, anti-nausea meds, charges for the infusion and monitoring…It’ll be a lot more than that.
As the FDA notes in its press release, production of Perjeta is currently limited due to a technical issue at the Genentech manufacturing plant. Meanwhile, investigators, doctors and patients will have to sort out the relative value of this drug, on top of the others – including pills – for Her2+ disease.
My opinion is not quite formed on this new antibody. The FDA’s decision was based on results from one trial of 808 patients, half of whom didn’t get the experimental drug. Accrual began in 2008; its broad clinical effects, and long-term toxicities, can’t be established yet. It may be, ten years from now, that Perjeta will be used routinely in patients with other, Her2+ kinds of cancer. Or it may be a toxic bust. How (and if) we’ll test and compare different doses of Perjeta and potential combinations with other drugs, small pills and traditional chemotherapies – which are many – is not clear. You could, for example, combine one or both of the antibodies with a drug like Lapatinib (Tykerb), that inhibits Her2-triggered growth signals inside the cell.
The problem is that oncologists, and facilities including academic centers where revenue is generated by giving drugs by infusion, now have a huge financial incentive to give the Herceptin-Perjeta-Taxotere regimen. This regimen is approved for first-line treatment of metastatic, Her2+ breast cancer; you don’t have to have “failed” another regimen, as was required for the EMILIA trial. As I understand this approval, an oncologist seeing a woman with recurrent or metastatic Her2+ breast cancer could, immediately, prescribe the 3-drug combination.
It’s impressive that the CLEOPATRA folks included an 89 year old patient in the study. But at some point, you have to wonder where we might draw lines. I’ve no answers on that.
All for now, maybe for the week,
ES
Leave a Reply