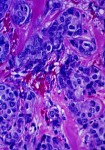
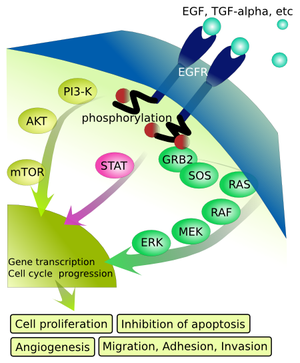
The new agent is a hybrid of an old monoclonal antibody, Herceptin, that’s chemically attached to DM1, a traditional kind of chemotherapy. The preliminary results of this randomized trial are encouraging. …It’s hard to know how this promising, likely expensive, intravenous drug will fit in with others for patients with Her2+ breast cancer.
Posted in Breast Cancer, cancer treatment, Medical News, PharmacologyTagged ASCO, EMILIA trial, Her2+ breast cancer, Maytenus serrata, metastatic breast cancer, T-DM1, targeted therapy, Trastuzumab Emtansine